EXCEED ET (NCT05482971): a Phase 2b, single-arm, multicenter study designed to evaluate the effectiveness and safety of ropeginterferon alfa-2b-njft in adult patients with essential thrombocythemia (ET).1,2
Ropeginterferon alfa-2b-njft is an investigational therapy that is not yet approved by any regulatory authority for the treatment of ET.1
PharmaEssentia is the sponsor of the EXCEED ET study.1
Now recruiting: Phase 2b1,2
What is the EXCEED ET study?1
PharmaEssentia is conducting a Phase 2b clinical research study in adult patients with ET. The purpose of the EXCEED ET study is to evaluate the effectiveness and safety of ropeginterferon alfa-2b-njft, a monopegylated IFN alfa-2b, in ET.
Study design (N=64)
- Adult patients with a confirmed diagnosis of ET according to the 2016 WHO criteria
- Patients who are IFN treatment–naïve, cytoreductive treatment–naïve, or pre-exposed to hydroxyurea (HU) and/or anagrelide (ANA)
- See additional eligibility criteria
4 weeks
Screening period
4 weeks*
Titration period
Day 0 (250 mcg)
Day 14 (350 mcg)
Day 28 (500 mcg)
12 months†
Up to 2 years†
Core treatment period
Extension period
Ropeginterferonalfa-2b-njft
500 mcg SC Q2W
Up to 4 weeks after last treatment visit
Follow-up period
Primary endpoint
- Durable response rate (PLT count ≤400 x 109/L and WBC count <10 x 109/L for ≥80% of biweekly measurements for a consecutive 32-week period during the 52-week core study treatment period)
Key secondary endpoints
- Clinical response per modified ELN criteria
- Hematologic response (PLT count ≤400 x 109/L and WBC count <10 x 109/L)
- Duration of peripheral blood count remission response
- Incidence of disease-related thromboembolic events
- Disease progression to post–ET MF and secondary AML
- Change of JAK2, CALR, and MPL allelic burden and other molecular abnormalities over time
Exploratory endpoints
- Symptomatic improvement (MPN-SAF TSS) and quality of life (EQ-5D-3L)
- Bone marrow histological remission
- Further genetic and molecular markers
What is essential thrombocythemia (ET)?
ET is a rare myeloproliferative neoplasm characterized by several genetic mutations, which trigger thrombocytosis and the potential for thrombotic and hemorrhagic complications.3,4 The driver mutations known to cause the overproduction of hematopoietic cells include JAK2, CALR, and MPL, which can lead to an increase in blood counts, including the number of PLTs, and in some cases, WBCs.3-6 Current therapies used to treat ET may include ANA (an FDA-approved therapy used to reduce PLT production), HU, and IFN alfa.7
What is ropeginterferon alfa-2b-njft?
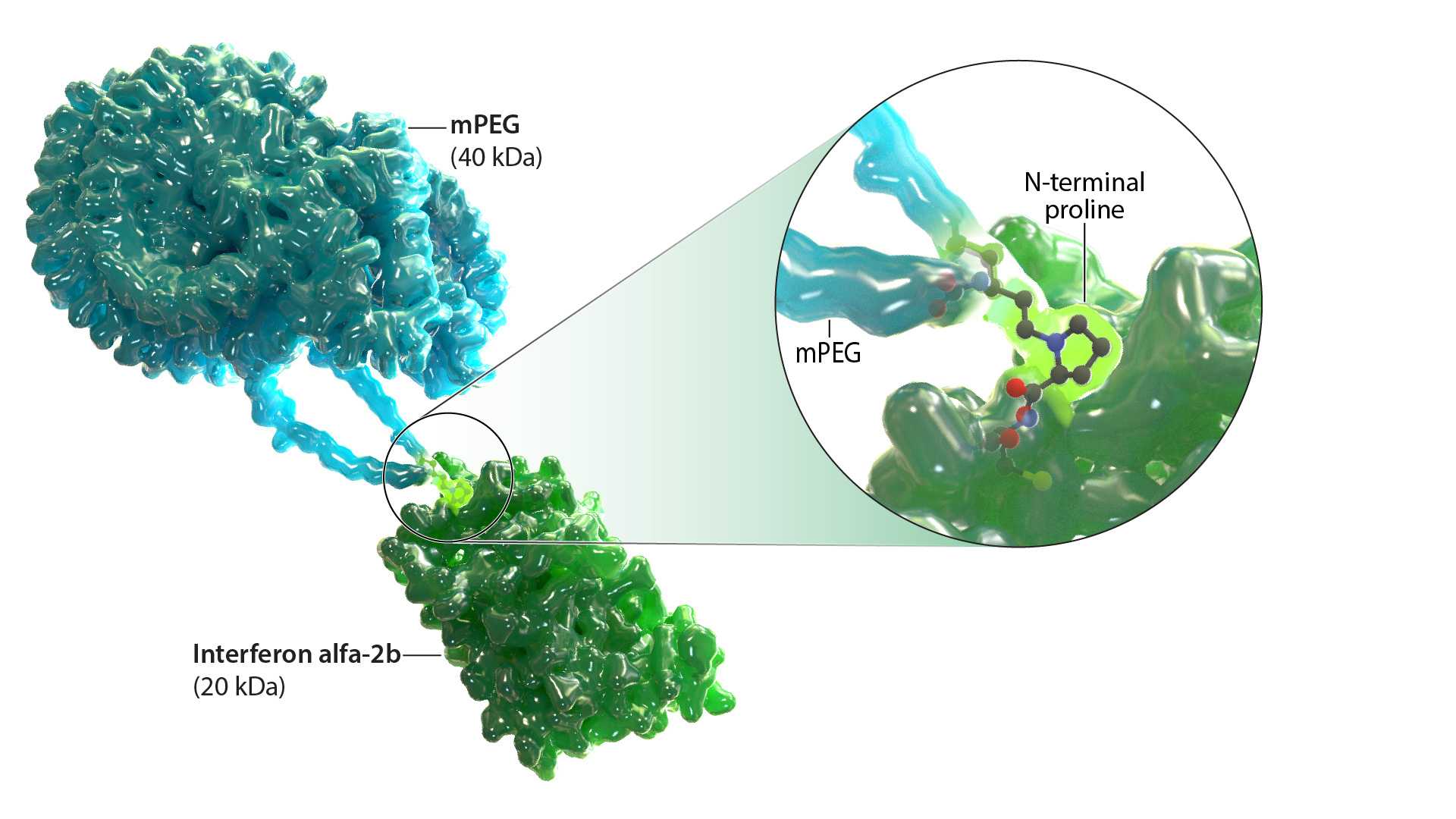
Ropeginterferon alfa-2b-njft is a monopegylated IFN alfa-2b that selectively targets JAK2 mutant hematopoietic stem and progenitor cells.8,9 It is an investigational therapy that is not yet approved by any regulatory authority for the treatment of ET.1
Who is eligible for EXCEED ET?1
≥18 years of age
Confirmed diagnosis of ET according to the 2016 WHO criteria
Prior treatment with HU and/or ANA
Cytoreductive treatment–naïve
IFN treatment–naïve
Adequate hepatic function (bilirubin ≤1.5 x ULN, PT ≤1.5 x ULN, albumin >3.5 g/dL, ALT ≤2.0 x ULN, AST ≤2.0 x ULN)
CrCl ≥40 mL/min
PLT count >450 x 109/L
For additional information on eligibility criteria, visit ClinicalTrials.gov (trial identifier: NCT05482971).2
Where is EXCEED ET being conducted?
Find out if there’s a study site in your area using the table below. For additional information, please contact us by emailing clinicaltrials@pharmaessentia-us.com or call 1-800-999-2449.
- Juravinski Cancer Centre
- Hamilton, ON
- Princess Margaret Hospital
- Toronto, ON
- Yale University School of Medicine - Yale Cancer Center
- New Haven, CT
- John Theurer Cancer Center at Hackensack UMC
- Hackensack, NJ
- Montefiore Medical Center
- Bronx, NY
- Fox Chase Cancer Center
- Philadelphia, PA
- The Winship Cancer Institute Emory University
- Atlanta, GA
- East Carolina University
- Greenville, NC
- MD Anderson Cancer Center
- Houston, TX
- University of Texas Health Science Center at San Antonio
- San Antonio, TX
- University of Virginia - Emily Couric Cancer Center
- Charlottesville, VA
- Mayo Clinic - Scottsdale
- Scottsdale, AZ
- Marin Cancer Care
- Greenbrae, CA
- Tulane University Medical Center
- New Orleans, LA
- University of Utah
- Salt Lake City, UT
- University of Michigan
- Ann Arbor, MI
- Washington University School of Medicine - Division of Oncology
- St. Louis, MO
- Cleveland Clinic
- Cleveland, OH
How do I refer patients to EXCEED ET?
To refer a patient, please contact our study information center at 1-800-999-2449.
To become an investigator or conduct the EXCEED ET study at your institution, please contact our Medical Information team at medinfo@pharmaessentia-us.com.